Abstract
Background
A combination of drugs including a corticosteroid, a proteosome inhibitor, an immunomodulatory drug, and more recently, an anti-CD38 monoclonal antibody are used for the treatment of newly diagnosed Multiple Myeloma (NDMM). The most recent National Comprehensive Cancer Network (NCCN) guidelines lists various options with a general recommendation of using a triplet regimen (two drug classes and corticosteroid) as the standard therapy and a doublet regimen in patients with poor performance status or who are frail.1 For primary therapy in non-transplant candidates, the NCCN guidelines recommend bortezomib/lenalidomide/dexamethasone (VRd), daratumumab/lenalidomide/dexamethasone (DRd), carfilzomib/lenalidomide/dexamethasone (KRd), ixazomib/lenalidomide/dexamethasone (NRd), daratumumab/bortezomib/melphalan/prednisone (D-VMp), daratumumab/cyclophosphamide/bortezomib/dexamethasone (D-VCd), bortezomib/dexamethasone (Vd), bortezomib/cyclophosphamide/dexamethasone (VCd), cyclophosphamide/lenalidomide/dexamethasone (CRd), carfilzomib/cyclophosphamide/dexamethasone (CKd), and lenalidomide/low-dose dexamethasone (Rd).
This study examines the first-line (1L) treatment patterns, including drugs used and duration of the treatment.
Methods
>
A cohort of patients diagnosed and newly treated for multiple myeloma from 2018 through October 2020 was identified from the Optum Labs Data Warehouse, which includes de-identified claims data for commercially insured and Medicare Advantage enrollees. Eligible members have ≥1 medical claim with a diagnosis code for Multiple Myeloma (ICD10 code: C90.0*) between 1/1/2018 and 10/31/2020 ('diagnosis index date') and ≥1 claim for systemic treatment (oral or injected drugs) within 180 days following diagnosis index date; the first drug claim was the 1L start date. Patients with claims indicating receipt of stem cell transplantation were excluded. Continuous enrollment with medical and pharmacy benefits 180 days prior to diagnosis index date was required and patients were followed through 30 days after the first claim for systemic therapy. The final study population of newly diagnosed MM (NDMM), were required to have no claims for MM or other cancers prior to the diagnosis index date.
NDMM regimens were defined as those drugs received within the first thirty days of the 1L start date. Duration of treatment or real-world time to discontinuation (rwTTD) use was defined as number of days from 1L start date to last date of drug administration or prescription fill for the drugs in 1L regimen. We estimate rwTTD and account for censoring via SAS PROC LIFETEST.
Results
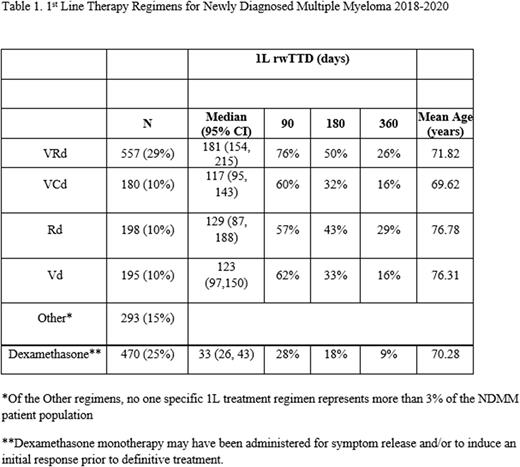
We identified 1,893 non-transplant NDMM patients who initiated treatment between 2018 - 2020. The mean age at diagnosis was 72 years and a similar proportion were male (50.9%) and female.
VRd and DRd were the preferred NCCN regimens for 1L in NDMM. 29% of 1L regimens in our sample were VRd (29%). Thirty percent of 1L regimens were either VCd (10%), Rd (10%), or Vd (10%). 25% of all regimens were single-agent dexamethasone. All other 1L regimens accounted for 15% of all NDMM 1L treatments. Excluding monotherapy dexamethasone regimens, the median rwTTD ranged between 117-181 days with 26% of VRd patients remaining treatment for a year. Monotherapy dexamethasone was the second most common 1L regimen and was brief in most cases, with 72% less than 3 months. About 61% of these patients went on to receive an apparent 2L regimen.
Conclusions
Consistent with guidelines for the treatment of non-transplant NDMM, the most common 1L regimens were triplets and doublets. Median rwTTD and percent of patients remained on 1L at 90, 180 and 360 days varied by regimen. Use of three drug regimens was common, representing 41% of all 1L regimens, the most common being VRd and VCd. Duration of 1L treatment, as measured by rwTTD, was the greatest for patients on lenalidomide-based regimens.
References: 1. The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf
Disclosures
Dacosta Byfield:Somalogic; Illumina: Other: I am employed at UnitedHealthGroup which received funding for a study that I work on.
Author notes
Asterisk with author names denotes non-ASH members.